File a Tier II Report Revision
Revisions are amendments of previously submitted Tier II reports used to correct inaccurate information. This guide will walk you through the process of filing a revision in Encamp.
Note that revisions correct information for activities that occurred during the reporting year of the original Tier II report. For example, an RY2023 Tier II report filed in January 2024 reflects information from January 1 to December 31, 2023. A new chemical brought on-site in January 2024 would not require a revision but would require an initial notification instead. Revisions would be used to correct any already-reported information for activities that occurred during 2023.
Steps to File a Revision
Step 1: Access the Chemicals Reports page
Click Chemicals in the left-hand navigation panel and you will be directed to the Reports page.
Step 2: Locate the Facility and Report
Use the search bar or filters to find the relevant facility
Ensure the facility has an ANNUAL report with a status of "Processing" or "Filing Complete"
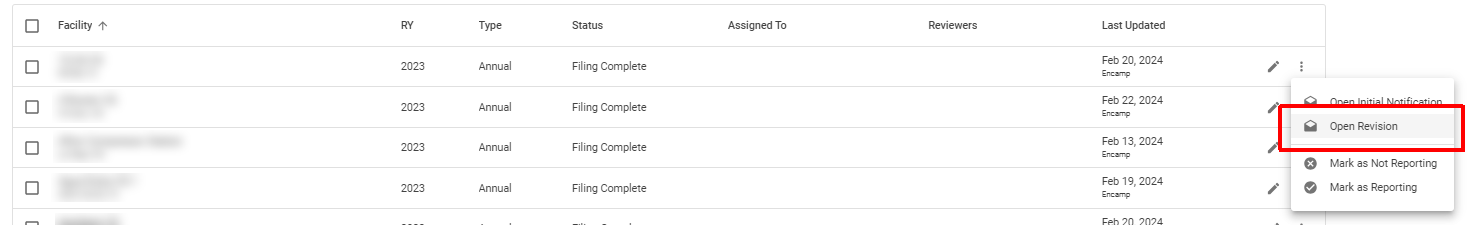
Step 3: Open a Revision
Click the three dots next to the submitted annual report
Select Open Revision
Step 4: Review and Update Report Information
You'll be taken to a page similar to the Tier II report page. Here you can correct any inaccurate information in the original report, such as chemical quantities, facility information, or contact details.
Note: Only make changes that correct information for the original reporting year. Do not include new information for the current year.
Any updates made to the report information will be reflected in subsequent reports for this facility (e.g., Initial Notifications or the following year's Tier II report).
Step 5: Submit the Revision
Resolve any flagged Compliance Issues
Add the verifier information and click Submit Report
After Submission
Once you submit the revision, Encamp handles the filing process automatically. The revision is submitted to the appropriate regulators in the required format for your jurisdiction. Unlike initial notifications or Tier II reports, revisions typically do not have associated fees.
All documentation related to the revision will be stored in the Documents section for the facility, ensuring easy access for future reference. The corrected information will be reflected in the facility's compliance status and will be used for any subsequent reports or data requests.
After submission, you can file additional revisions if needed, allowing you to maintain accurate compliance records throughout the year.
Important: Always ensure that revisions are necessary and accurate before submitting. Frequent revisions may attract regulatory scrutiny.